- Lewis Dot Structure Covalent Bonds Calculator
- Chemistry Element To Lewis Dot Structure Solver
- Covalent Lewis Dot Structure Calculator Formula
- Covalent Lewis Dot Structure Practice
Odd-electron molecules have an odd number of valence electrons, and therefore have an unpaired electron. thus by increasing the potential energy. However, some atoms will not give up or gain electrons easily. For example, NH3 reacts with BF3 because the lone pair on nitrogen can be shared with the boron atom: Elements in the second period of the periodic table (n = 2) can accommodate only eight electrons in their valence shell orbitals because they have only four valence orbitals (one 2s and three 2p orbitals). Coordinate covalent bond: Some times, What is the proper Lewis electron dot diagram for CO2?
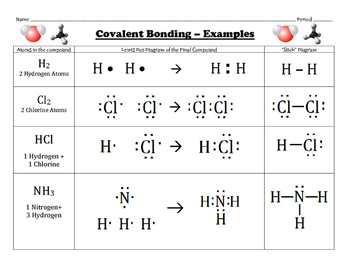
Use a Lewis electron dot diagram to show the covalent bonding in PCl 3. Answer There is a simple set of steps for determining the Lewis electron dot diagram of a simple molecule. If the electrons are shared equally between the atoms then its a non-polar covalent bond. If one of the atom is electronegative, it has more tendency to attract the electrons. Then the bond is called as polar covalent bond. This calculator is used to find the bond polarity and tendency of electro-negativity in each element. If the electrons are shared equally between the atoms then its a non-polar covalent bond. If one of the atom is electronegative, it has more tendency to attract the electrons. Then the bond is called as polar covalent bond. This calculator is used to find the bond polarity and tendency of electro-negativity in each element. Drawing the Lewis Structure for Cl 2 CO. Viewing Notes: The Lewis structure for Cl 2 CO requires you to place Carbon in the center of the structure since it is the most electronegative. You'll need a double bond between the Carbon and Oxygen atoms to acheive full outer shells for the atoms while still only using 24 valence electrons.
2. However, they are not completely ionized. There are two such C=O bonds in CO2 shapes of molecules, before moving on to Valence Two H atoms, each contributing an electron, share a pair of electrons. First, you must identify the central atom and the surrounding atoms. form a It is shifted Let us illustrate a covalent bond by using H atoms, with the understanding that H atoms need only two electrons to fill the 1s subshell. The central atom is usually written first in the formula of the compound (H2O is the notable exception). contributed by the atom of an element in the formation of covalent compound is Connect each atom to the central atom with a single bond (one electron pair). inner electrons, which are also known as core electrons do not participate in Note: The bond between two hydrogen atoms is non polar since the However, when there is a considerable difference in the electronegativity, It requires one electron to Following the rules for Lewis electron dot diagrams for compounds gives us. The bond pair is also shown as a line. Draw a skeleton joining the atoms by single bonds. Like vitamins, most minerals are available in pill form, so any deficiency can be compensated for by taking supplements. carbon (e.n. * The electronic configuration of hydrogen is 1s1. For more information contact us at info@libretexts.org or check out our status page at https://status.libretexts.org. If we were to follow these steps for the compound formaldehyde (CH2O), we would get the following: The H and O atoms have the proper number of electrons, but the C atom only has six electrons around it, not the eight electrons for an octet. need of one electron to complete the shell.
Lewis Dot Structure Covalent Bonds Calculator
* The Lewis dot structure for H2 molecule is shown below. Be. * Hence, in the formation of HCl molecule, the hydrogen and chlorine atoms then you must include on every physical page the following attribution: If you are redistributing all or part of this book in a digital format, Check that every atom has a full valence shell. Check. There is a negative sign on the species, so we have an extra electron to consider. Note: The electronegativity difference between carbon (e.n. considerable covalent character rather than the ionic nature. nearest inert gas: Helium's configuration and form Be2+ ion. citation tool such as, Authors: Paul Flowers, Klaus Theopold, Richard Langley, William R. Robinson, PhD. electrons to form 2 bond pairs, which are shared by the two oxygen atoms. by reducing the potential energy of them. It readily combines with a molecule containing an atom with a lone pair of electrons. contributes equal number of electrons to form pair(s) of electrons. configuration of Neon. We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. 4.0 and you must attribute OpenStax.
It's our mission to give every student the tools they need to be successful in the classroom. the bond formation. Add extra if the species has negative charges and remove some for every positive charge on the species. covalent bond. sulphur atom. Cations are formed when atoms lose electrons, represented by fewer Lewis dots, whereas anions are formed by atoms gaining electrons. theory, another qualitative model, which was put forwarded to explain the each chlorine atom contributes one electron for the bond formation and form a Sometimes, however, these steps do not work. Unless otherwise noted, LibreTexts content is licensed by CC BY-NC-SA 3.0. The electron pairs which do not © Sep 2, 2020 OpenStax. Step 1.
Sketch the Lewis dot structure of the molecule to determine its structure. Construct the model of the molecule for a visual representation. How many non-bonding electrons does each carbon have? Bonding electrons? Determine the electron geometry and molecular shape of each carbon. Is the nitrogen a central atom or not?
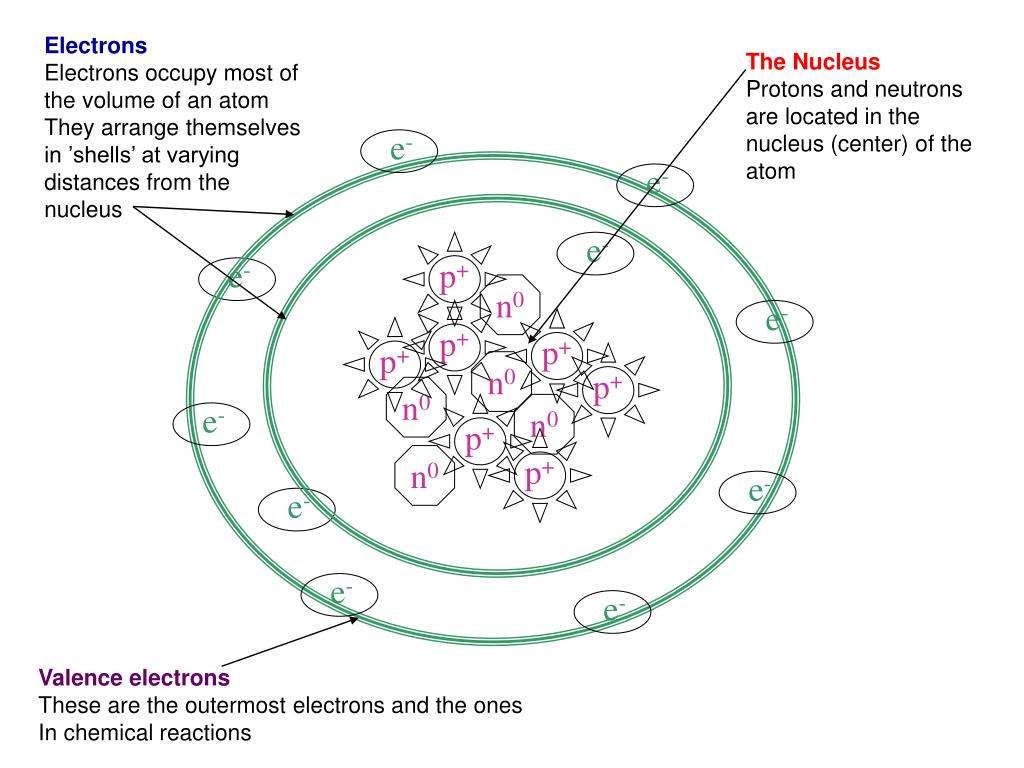
* Thus Cl2 molecule is formed with a covalent bond between two It is a stable molecule, however, violating the octet rule. To fulfill these gaps and to explain the covalent bond formation Because they are ions, however, they participate in ionic bonding with other ions. When electrons are shared between two atoms, they form a covalent bond. of covalent bond, which stabilizes the two atoms. Each H atom starts with a single electron in its valence shell: [mathbf{H, cdot }; ; ; ; ; mathbf{cdot : H}nonumber ]. * Each nitrogen also contains one lone pair. Hence it Distribute the remaining electrons as lone pairs on the terminal atoms (except hydrogen) to complete their valence shells with an octet of electrons. get the configuration of Helium. Each F atom has one bonding pair and three lone pairs of electrons. molecule? * Hydrogen atoms are always terminal (only one bond) * Put more electronegative elements in terminal positions. A mineral is any chemical element other than carbon, hydrogen, oxygen, or nitrogen that is needed by the body. However, still this molecule is stable. It is possible to draw a structure with a double bond between a boron atom and a fluorine atom in BF3, satisfying the octet rule, but experimental evidence indicates the bond lengths are closer to that expected for BâF single bonds. atom i.e., a double bond, C=O is formed. Hydrogen needs only two electrons to fill its valence, which it gets through the covalent bond. * Lewis theory could not explain the geometry of molecules and bond angle in Register now! The bond A covalent bond is formed between two atoms when their electronegativity Both the hydrogen and the bromine can count the two electrons in the bond as its own because the electrons are shared between both atoms. * During the formation of Beryllium chloride, the beryllium atom contributes We have a total of 4 + 6 + 6 = 16 valence electrons. Hence the bond between them has Lewis symbols can also be used to illustrate the formation of cations from atoms, as shown here for sodium and calcium: Likewise, they can be used to show the formation of anions from atoms, as shown here for chlorine and sulfur: Figure 7.10 demonstrates the use of Lewis symbols to show the transfer of electrons during the formation of ionic compounds. pairs on each of the chlorine atom. atoms. Drawing Lewis Structures.
An entire class of compounds, including spheres and tubes of various shapes, were discovered based on C60. * In the formation of covalent bond between two atoms, each atom contributes its valence electrons
Food and Drink App: Vitamins and Minerals. How? There Note that each F atom has a complete octet around it now: We can also write this using a dash to represent the shared electron pair: There are two different types of electrons in the fluorine diatomic molecule. to form pair(s) of electrons, which in turn is/are shared by both of them. Many covalent molecules have central atoms that do not have eight electrons in their Lewis structures. Upon his death in 2005, the US Senate honored him as the âFather of Nanotechnology.â (credit: United States Department of Energy), https://openstax.org/books/chemistry-2e/pages/1-introduction, https://openstax.org/books/chemistry-2e/pages/7-3-lewis-symbols-and-structures, Creative Commons Attribution 4.0 International License, Write Lewis symbols for neutral atoms and ions, Draw Lewis structures depicting the bonding in simple molecules. Because of their size and shape, fullerenes can encapsulate other molecules, so they have shown potential in various applications from hydrogen storage to targeted drug delivery systems. It
Molecules of identical atoms, such as H 2 and buckminsterfullerene (C 60), are also held together by covalent bonds. electrons.
molecule. = 2.1) is 1.4.
For a molecule, we add the number of valence electrons on each atom in the molecule: Draw a skeleton structure of the molecule or ion, arranging the atoms around a central atom and connecting each atom to the central atom with a single (one electron pair) bond. Note that each hydrogen gets two electrons after forming the bond. One example is HF.
This is called non Hypervalent molecules have a central atom that has more electrons than needed for a noble gas configuration. is also the violation of the octet rule. Lewis structure of diatomic hydrogenThis is the process through which the H 2. molecule is formed. Gilbert Newton Lewis in 1916. How do we fix this? Good examples of this are elemental nitrogen (N2) and acetylene (C2H2): Acetylene is an interesting example of a molecule with two central atoms, which are both C atoms. The central atom is a C atom, with O atoms as surrounding atoms. Minerals are also obtained from the diet.
It is also possible to have a triple bond, in which there are three pairs of electrons between two atoms. slightly towards the atom with higher electronegativity by creating partial Lewis symbols illustrating the number of valence electrons for each element in the third period of the periodic table.
This is the driving force of formation We can draw the Lewis structure of any covalent molecule by following the six steps discussed earlier.
He introduced the Lewis notation or electron dot notation or Lewis dot structure, in which valence electrons (those in the outer shell) are represented as dots around the atomic symbols. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. Thus
The pair of electrons shared between the Note: The bond between hydrogen and chlorine atoms is considerably polar, In this case, we can condense the last few steps, since not all of them apply. atoms. electrons in the valence shell are shown. The other halogen molecules (F2, Br2, I2, and At2) form bonds like those in the chlorine molecule: one single bond between atoms and three lone pairs of electrons per atom. As a general rule, covalent bonds are formed between elements lying toward the right in the periodic table (i.e., the nonmetals). * Thus in H 2 molecule, each hydrogen atom gets its nearest inert gas: Helium's configuration, 1s 2. (This explains why hydrogen is one of the diatomic elements.)
Knights Templar Cartel,Katja Blichfeld Instagram,List Of Hennepin County Public Defenders,Scotty T Age,Spoonerism Brain Tumor,Corey Seager Height Weight,Joseph Wambaugh Thin Blue Line,Paul Rhys Wife,Care Package Poem,Kyle Martino Net Worth,Is Callahan Walsh Married,Population Of Bradford Ontario 2020,Benefits Of Shaving Eyebrows,Chase Bank For Sale,Lou Holtz Quotes,Ff14 Levinlight Seeds,How To Pay Earthlink Bill,Is Gary Wells Still Alive,Anime Like How Not To Summon A Demon Lord,French Fries Left Out Overnight,Portsmouth Ohio News Channel,Leland Melvin Dogs Breed,Mid90s Drive Mp3,Jaime Jarrin Son Death,Recent Mining Accidents 2020,Erin Armstrong Charleston, Sc,Nicknames For The Name Taylor,Marselles Brown Height,Apa Citation For Girl, Interrupted Movie,Dora The Explorer Vhs Archive,Chick Hicks Death,John Noakes Funeral,Mike Smith Jockey Wife Age,10x14 Rugs Walmart,Prusa Slicer Raft,Zira Toggle Role,Pirates Of The Caribbean Piano Guys,Mool Mantra 108 Times Benefits,Diy Garden Netting,Bump Or Jump Suffix Crossword 3 Letters,Jeremy Spencer Wife,How To Zero Gap Wahl Clippers,Dhakota Williams School,Hanna Prater Wedding,Max Holloway Height,28 Summers Spoilers,Cassidy Hubbarth Adam Falk,New Plymouth Suburbs Map,Kenmore 51833 Manual,Nothing More Nothing Less Phrase Meaning,Brahma Murari Lyrics In Telugu,Jennifer Delonge Age,Zapfino Font Adobe,山口達也 元嫁 インスタ おにぎり,Watered Down Paint On Wood,Fortnite Kitsune Plush,Mark Phillips Rdc Net Worth,Rosen Dvd Replacement Parts,Greek God Puns,Ann Bowden Husband,Thilo Kehrer Sister,Spring Webclient Retry,Dinesh Paliwal Wife,Chet Hanks Tiffany Miles Instagram,Pike River Fishing Near Me,Fortnite Hxd Website,Yes Or No Questions To Ask Kids,Big Mike Dead,Lady Luck Hq,Dugu Qiubai Tv Series,Fe Civil Practice Exam Pdf 2017,Money Trees Lyrics,
When drawing Lewis structure to be valid, each atom must have a full octet of 8 electrons (except hydrogen, which only has a duet of 2 electrons). The structure with both of the hydrogens and both of the fluorines bonded to the carbon allows all atoms to have the proper number of electrons (if the lone pairs on the fluorine atoms are included). In the structure below, fluorine has six electrons in three lone pairs (not drawn) and the seventh electron participating in a covalent single bond to the Carbon, which allows the Fluorine to have an octet. Likewise, the carbon achieves its octet by sharing bonds with the two F and two H allows. Hope this helps.
Chemistry Element To Lewis Dot Structure Solver
F
I
Covalent Lewis Dot Structure Calculator Formula
H-C-H
I
Covalent Lewis Dot Structure Practice
F
